EAHAD Projects
EUHASS is a pharmacovigilance program to monitor the safety of treatments for people with inherited bleeding disorders in Europe.
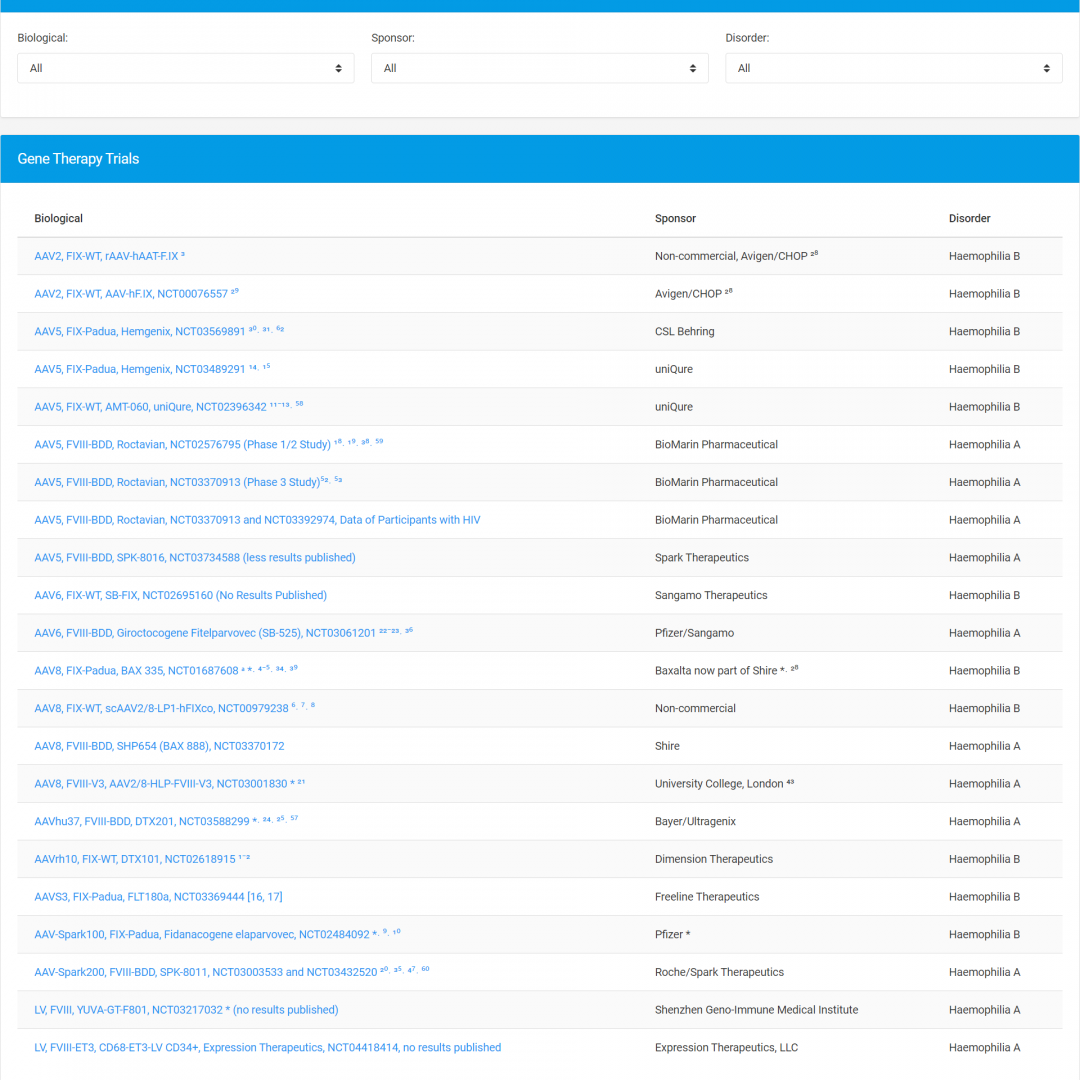
The EAHAD Gene Therapy Clinical Trials Database is user-friendly, publicly accessible database of the latest gene therapy results, with a focus on safety issues.
There are 409 known haemophilia centres in Europe shown on the map. The size and services vary enormously with some centres caring for more than 350 persons whilst others care for less than five.
The intention of this project is to gather single gene variant databases involved in clinical bleeding disorders, principally haemophilias A and B and von Willebrand disease, and other rarer coagulation factor variants.